ABSTRACT
Objective: This study aimed to investigate the potential parameters associated with imaging progression on chest CT from coronavirus disease 19 (COVID-19) patients.
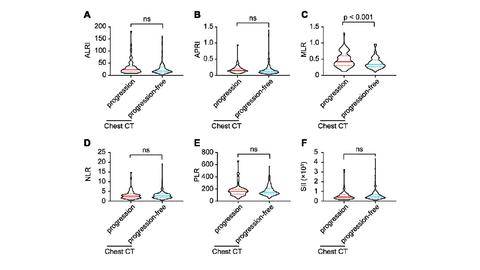
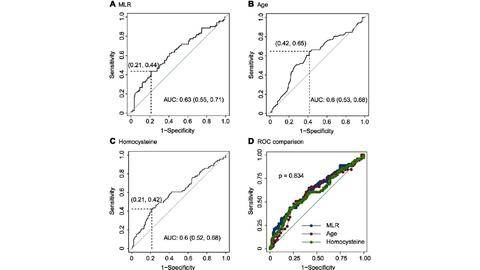
Results: The average age of 273 COVID-19 patients enrolled with imaging progression were older than those without imaging progression (p = 0.006). The white blood cells, platelets, neutrophils and acid glycoprotein were all decreased in imaging progression patients (all p < 0.05), and monocytes were increased (p = 0.025). The parameters including homocysteine, urea, creatinine and serum cystatin C were significantly higher in imaging progression patients (all p < 0.05), while eGFR decreased (p < 0.001). Monocyte-lymphocyte ratio (MLR) was significantly higher in imaging progression patients compared to that in imaging progression-free ones (p < 0.001). Logistic models revealed that age, MLR, homocysteine and period from onset to admission were factors for predicting imaging progression on chest CT at first week from COVID-19 patients (all p < 0.05).
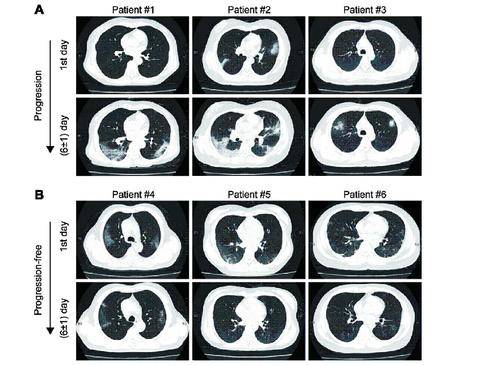
Conclusion: Age, MLR, homocysteine and period from onset to admission could predict imaging progression on chest CT from COVID-19 patients. Methods: The primary outcome was imaging progression on chest CT. Baseline parameters were collected at the first day of admission. Imaging manifestations on chest CT were followed-up at (6±1) days.